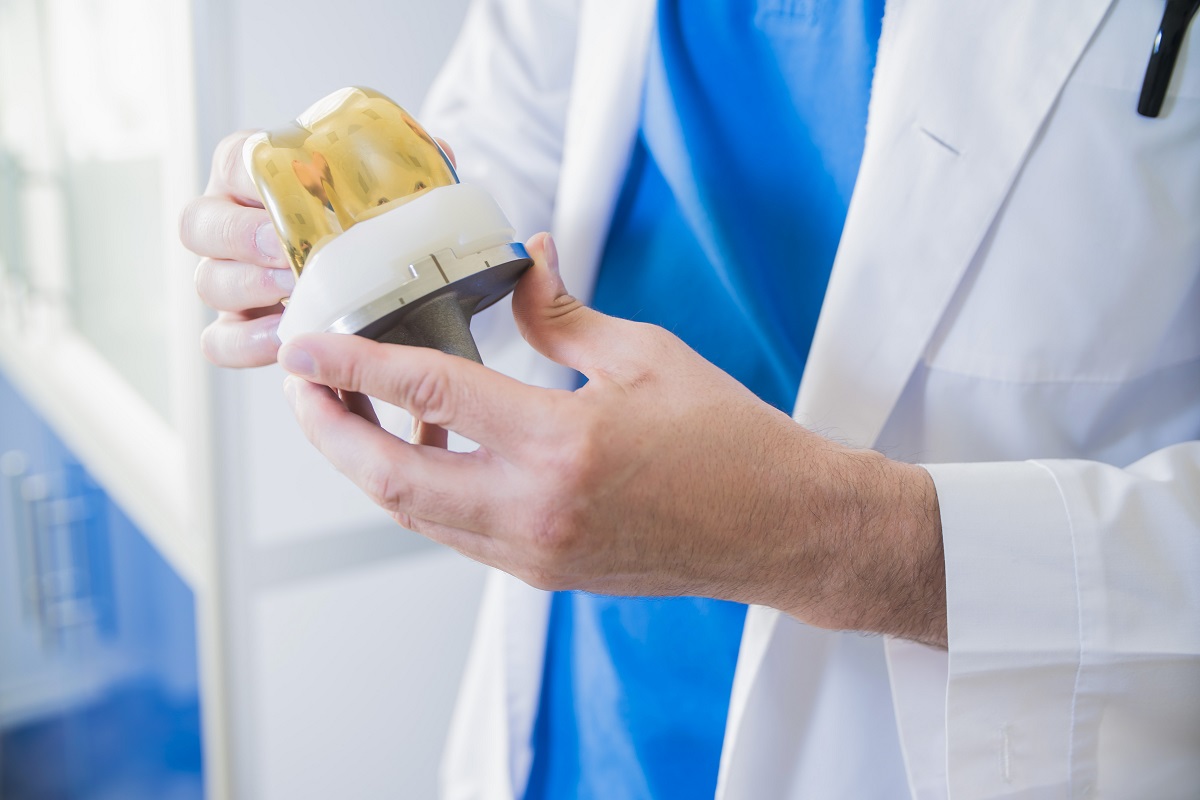
Francesco Robotti, Technology Business Development Manager at Lincotek Medical, looks at how surface treatment for the orthopedic devices market is being transformed and speculates on developments in the period to come.
April 20, 2021 | When we look at surface treatment innovations, there are two trends in orthopedic devices that are really driving change. The first is the need to achieve the same outcomes for a lower cost. The second is to achieve those same outcomes with quicker delivery and time to market – which requires a reduction in supply-chain complexity.
It’s not so much a question of finding a totally innovative material which fixes performance, but rather a constant improvement in processes.
When it comes to the complex nature of the supply chain, for instance, you can match improved osseointegration performances with a number of different porous structures and materials and a combination of different manufacturing steps. However, there’s a greater appreciation today of 3D printing to simplify everything consistently under one roof – from drawing to final part.
Here at Lincotek Medical, we’re investing in R&D, as you’d expect. Particularly with regard to the special processes that sustain production: 3D printing of implants; PVD; DLC; electrochemical treatments and coatings; and antibacterial surface treatments. In relation to 3D printing, we’re especially interested in the application to mini-invasive surgery and high fatigue-strength demand. We’ve made a lot of advances in this area.
There is also substantial interest from OEMs in electrochemical-based Brushite coatings. This material is a precursor to hydroxyapatite (HA) and shows rapid dissolution and reprecipitation to HA. Many in the spine community are exploring ways to commercialize this technology with us.
Concern over ion release from CoCrMo alloy is driving exploration and commercialization of wear-resistant coatings, such as PVD of TiN or TiNbN. In fact, we submitted a Master File to the FDA last August. We are getting a lot of calls from OEMs in the knee and ankle space, who want to offer coatings to mitigate potential ion release.
Our Thermal Plasma Spray can be applied on machined or forged metal parts to enable osseointegration. This can also be achieved by applying porous coating on implants made of bioinert materials, such as PEEK or cereamic. We offer Brushite coatings – developed through wet chemistry – for Additive Manufactured parts. What’s more, we can provide TiNbN PVD as a wear-resistant, ion-release blocking barrier for joint components.
So, what of the future? I foresee trends in the next five years towards personalization and miniaturization, as well as price pressure and shorter delivery times. There will also be greater demand for antibacterial surface treatments, navigated and robotic surgery and single-use disposable instruments. We will be responding on multiple fronts by advancing our 3D printing capability and printed material performances, while also using AI software and big data analysis to sustain process improvement.
We’ll continue to place an emphasis on process efficiency to reduce costs. This could involve smart quality controls, along with lean and fast processes – from inception to delivery. We are also working on anti-fouling surfaces that are not based on antibiotics.
It’s certainly an exciting time and Lincotek Medical is right at the heart of the developments within the sector.